Looking for an ACP? Have No Importer in Japan? We are Here to Help You!
Food, Beverages, Kitchen Utensils, and Infants' Toys
How to Import Food, Beverages, Kitchen Utensils, and Infants' Toys
Food Sanitation Act
Food, beverages, kitchen utensils, and infants' toys, and other products that can be taken into people's mouth are regulated by Food Sanitation Act in Japan.
Food Import by Non-Resident Importers

By taking responsibility for compliance with the Food Sanitation Act and other related regulations, an ACP enables non-resident importers to import these products. When importing, it is necessary to submit a Notification of Importation of Food, undergo ingredient inspection, and ensure product labeling. Therefore, it is important to select an ACP capable of handling these tasks.
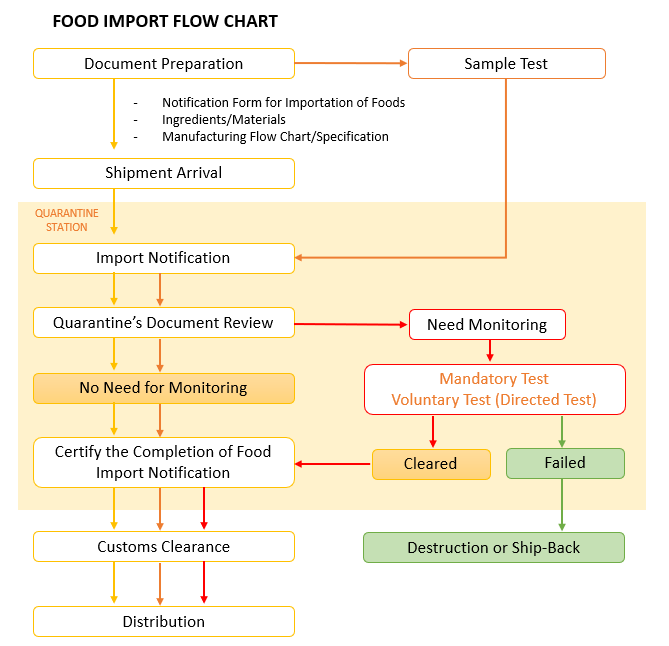
Food Import Flow
Labeling
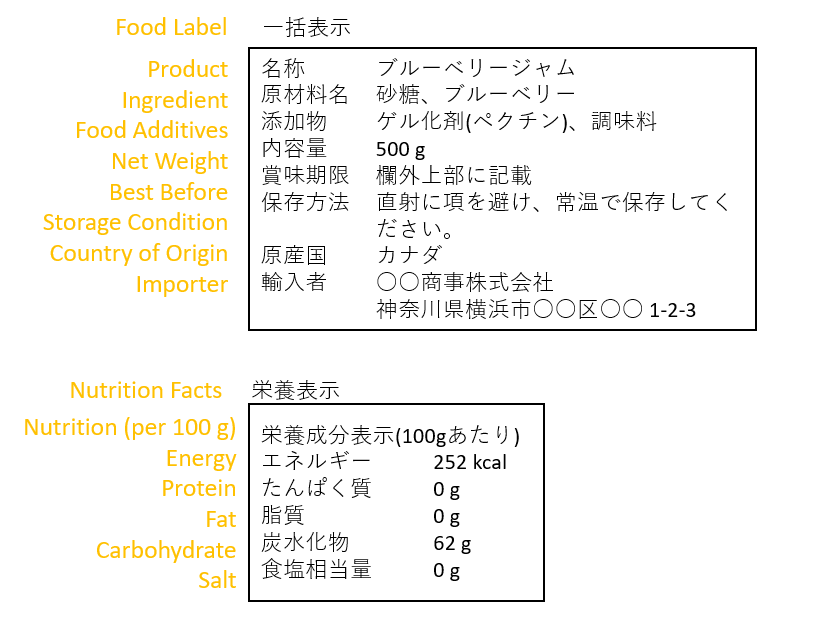
Food labeling is extremely important for the safety of consumers. It is mandatory for the manufacturers or importers to state the ingredients, expiration date, nutritional information, etc., on the packages of food and beverages. For imported food products, the importer is responsible for labeling, but overseas companies cannot state their information as the importer. Therefore, ACP's company information will be included as the responsible entity.
Furthermore, the display of recycling marks on packaging is also mandatory.
All these require good knowledge and expertise about food labeling, so it is very important that you appoint an appropriate ACP to help you import food products.


Expected Costs

Food Import Notification and Customs Handling Service :
50,000 ~300,000 JPY
Testing Costs : 20,000円~/substance
<PSE Act> Other Possible Concerning Laws
Juicers, coffee makers, blenders and other electric appliances with AC adapters are regulated by Electrical Appliances and Material Safety Act. Importers may need to experience a long process over months and millions yen investment in order to clear this regulation. See this page for more details.. See this page for more details.
<PMD Act and Health Promoting Act> Other Possible Concerning Laws
You are not allowed to state any medical or health-promoting effects about dietary supplements and other health food which have been imported as simple foods. In order to state such effects, you will need to submit clinical proofs and obtain approval from the applicable authorities subject to PMD Act or Health Promoting Act.
It's also important to note that some ingredients are only allowed to be used in pharmaceutical products.
Please visit this page to learn more about PMD Act.
Management of Best-Before Date

FBA (Fulfilment By Amazon) has special requirement for sellers of items that has best-before or expiration dates. Learn more about this here or contact Seller Central.
Contact Us for Any Questions
お気軽にお問合せください

Call Us at お電話でのお問合せ
Contact us 24 hours via the form below.
問合せフォームは24時間受け付けております。お気軽にご連絡ください。
Updates 新着情報
2025年10月12日の輸入申告項目の追加に従い、記載内容を一部更新しました。
2023年10月1日の関税法基本通達改正に従い、記載内容を一部更新しました。
Our column page “Customs Specialist Eyes” is updated.
コラムを更新しました。
Our column page “Customs Specialist Eyes” is updated.
コラムを更新しました。
We’re now on Amazon SPN/Service Provider Network.
当ページ運営会社が、Amazon SPN(サービスプロバイダーネットワーク)に登録されました。
ホームページを公開しました