Looking for an ACP? Have No Importer in Japan? We are Here to Help You!
Cosmetics, Medicine, and Health Care Items
How to Import Cosmetics, Medicine, and Health Care Items
Pharmaceutical and Medical Device Act(PMD Act)
Cosmetics, medicines, and healthcare products are controlled by Pharmaceutical Act. In order to import these items, you need applicable licenses, which can only be granted to domestic companies.
Import and Sales by Non-Resident Companies

A foreign company can import pharmaceutical products by appointing a properly licensed Japanese company as their ACP. However, some products require a sales license that a foreign company cannot obtain. This means that while these products can be imported, they cannot be sold directly by the foreign company. Instead, a licensed Japanese company must purchase and sell these products.
PMD Act Scope
Here are typical examples of items that are subject to Pharmaceutical Act.
【Cosmetics】
PMD Act defines ‘Cosmetic’ as ‘any item having mild effects on the human body that is rubbed, spread, or otherwise applied in a similar manner for the purpose of cleansing, beautifying, or enhancing the attractiveness of the human body, to change physical appearance, or to maintain skin or hair in a healthy condition’.
Example : Soap, toothpaste, shampoo, conditioner, skin-care products, make-ups, etc.
【Quasi Drugs】
PMD Act defines quasi drugs as low-risk products aimed to
1. Prevent nausea and other discomfort
2. Prevent heat rash, soreness, etc.
3. Encourage hair growth or removing hair
4. Exterminate and prevent mice, flies mosquitoes, fleas, etc.
【Drugs】
Subject to PMD Act, the term "drugs" refers to the following substances:
1) Substances listed in the Japanese Pharmacopoeia.
2) Substances (other than quasi-drugs and regenerative medicine products), which are intended for use in the diagnosis, treatment, or prevention of disease in humans or animals, and which are not equipment or instruments, including dental materials, medical supplies, sanitary materials, and programs.
3) Substances (other than quasi-drugs, cosmetics or regenerative medicine products) which are intended to affect the structure or functions of the body of humans or animals, and which are not equipment or instruments.
Examples : Prescription drugs, or OTC drugs including cold relief, digestive medicines, eye drops, etc..
【Medical Devices】
PMD Act defines medical devices as "machinery or appliances intended for diagnose, remedy or prenvetion of human or animal diseases, or influence in any way human or animal body structures or functions.
Examples : glasses, contact lenses, clinical thermometers, hearing aids, magnetic therapy equipment, electric massagers, etc.
あいうえおかきくけこさしすせそたちつてとなにぬねのはひふへほまみむめもやゃゆゅよらりるれろわ・を・んアイウエオカキクケコサシスセソタチツテトナニヌネノハヒフヘホマミムメモヤャユュヨララリルレロワ・ヲ・ン

Each category is classified in details by the functions and influence to the user bodies. The processes and licenses all vary by the classification. Please talk with your import partner to see if they have the applicable license and how you can start selling your products in Japan.
Labeling
Pharmaceutical items must come with labels.
Information required include usage instructions, warning, ingredients or materials, serial numbers, expiration date, importer details, or any other subjects requested per the class.
It is also important to carefully choose the marketing words and expressions, because each category has the limit on the expression allowed to describe products. This applies not only on the package and instructions, but also on the promotional materials and online pages.
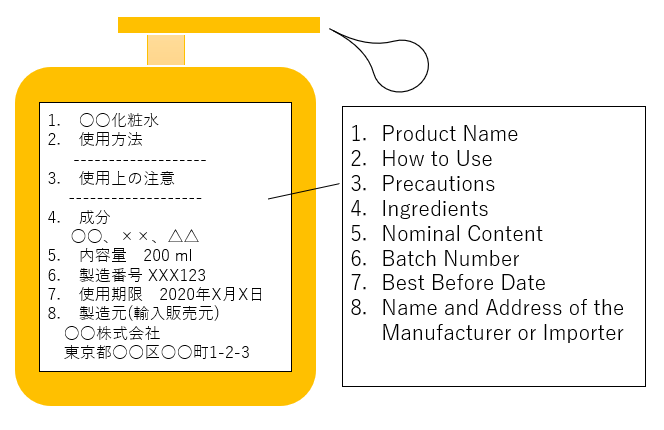
Example of Cosmetic Labeling
Import Flow
Pharmaceutical items are imported in the following manner.
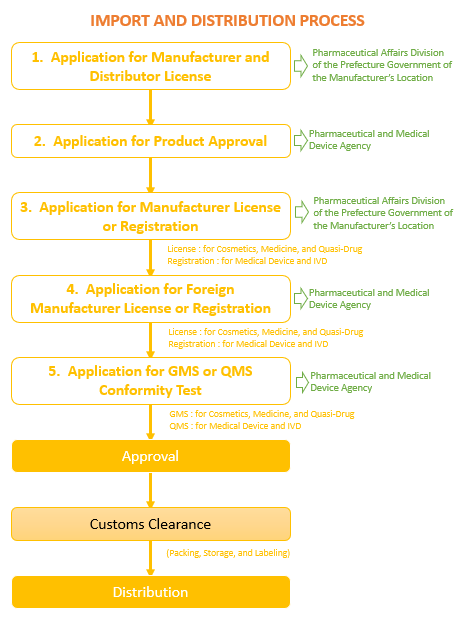
Expected Preparation Time and Costs

Obtaining import permission on pharmaceutical items is expected to involve the following costs and timeline, excluding marketing costs and time. Cosmetics and some simple medical devices are relatively easy, but others require expensive and long procedures to ensure safety.
Cosmetics : 50,000 JPY~/ 1 month~
Quasi Drugs : 1,000,000 JPY~/ 6 months~
Drugs : millions of JPY~/ over years~
Medical Device:100,000 JPY~ or 1,000,000 JPY~/ 6 months~
<PSE Act> Other Possible Concerning Laws
Electric massagers and other electric appliances with AC adapters are regulated by PSE Act(Electrical Appliances and Material Safety Act). In order to become ready to sell PSE items, sellers need to experience a long process of several months or more, costing millions of Japanese Yen. PSE process details are found here.
Retail License

License Certificate for Establishing Pharmacies)Some pharmaceutical items require retail license, in addition to manufacturing and importing.
It's important that your distributors have the necessary license to sell the products that you import to Japan.
| Class | Marketing Notification or License | |
|---|---|---|
| Cosmetics | Not Required | |
| Quasi-Drugs | Not Required | |
| Drugs | OTC and Switch OTC Drugs | Licenses for Store-Based Distribution |
| Other High-Class Drugs | License Certificate for Establishing Pharmacies | |
| Medical Device | Class I | Not Required |
| Class II, III, and IV | License for Retailing and Renting Medical Devices |
Notification and application for license can only be done by registered and qualified Japanese companies.
Contact Us for Any Questions
お気軽にお問合せください

Call Us at お電話でのお問合せ
Contact us 24 hours via the form below.
問合せフォームは24時間受け付けております。お気軽にご連絡ください。
Updates 新着情報
2025年10月12日の輸入申告項目の追加に従い、記載内容を一部更新しました。
2023年10月1日の関税法基本通達改正に従い、記載内容を一部更新しました。
Our column page “Customs Specialist Eyes” is updated.
コラムを更新しました。
Our column page “Customs Specialist Eyes” is updated.
コラムを更新しました。
We’re now on Amazon SPN/Service Provider Network.
当ページ運営会社が、Amazon SPN(サービスプロバイダーネットワーク)に登録されました。
ホームページを公開しました

